Nápady Atom Particles
Nápady Atom Particles. Some particles were last … Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … However, the term particle refers to any small object. We call them subatomic particles. Atoms are small units of matter which contain several particles;
Nejlepší Lesson 2 Subatomic Particles
Particle physics is a journey into the heart of matter. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. The crossword solver found 20 answers to the atomic particle crossword clue.We call them subatomic particles.
Enter the answer length or the answer pattern to get better results. The crossword solver found 20 answers to the atomic particle crossword clue. However, the term particle refers to any small object. Atoms are small units of matter which contain several particles; Click the answer to find similar crossword clues. Atomic particles atoms consist of three basic particles: Some particles were last …

However, the term particle refers to any small object. Particle physics is a journey into the heart of matter. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Click the answer to find similar crossword clues. Atoms are small units of matter which contain several particles; Atomic particles atoms consist of three basic particles:. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.

Atoms are small units of matter which contain several particles;. Every atom has a nucleus that bounds one or more electrons around it. We call them subatomic particles. Enter the answer length or the answer pattern to get better results. Click the answer to find similar crossword clues. Some particles were last … The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.

Atoms are small units of matter which contain several particles;. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Atomic particles atoms consist of three basic particles: Some particles were last … Every atom has a nucleus that bounds one or more electrons around it. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). We call them subatomic particles. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, …. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons.

The crossword solver found 20 answers to the atomic particle crossword clue. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). We call them subatomic particles. Every atom has a nucleus that bounds one or more electrons around it. The crossword solver found 20 answers to the atomic particle crossword clue. Each individual atom consists of smaller particles—namely, electrons and nuclei.
Particle physics is a journey into the heart of matter. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Atomic particles atoms consist of three basic particles: We call them subatomic particles. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). However, the term particle refers to any small object.

Each individual atom consists of smaller particles—namely, electrons and nuclei. Every atom has a nucleus that bounds one or more electrons around it. Enter the answer length or the answer pattern to get better results. Atoms are small units of matter which contain several particles; Each individual atom consists of smaller particles—namely, electrons and nuclei. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Particle physics is a journey into the heart of matter. However, the term particle refers to any small object.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Every atom has a nucleus that bounds one or more electrons around it. Particle physics is a journey into the heart of matter. Each individual atom consists of smaller particles—namely, electrons and nuclei. Click the answer to find similar crossword clues. Enter the answer length or the answer pattern to get better results. The crossword solver found 20 answers to the atomic particle crossword clue. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form.

The crossword solver found 20 answers to the atomic particle crossword clue. . Enter the answer length or the answer pattern to get better results.

Each individual atom consists of smaller particles—namely, electrons and nuclei. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Some particles were last … We call them subatomic particles. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Atomic particles atoms consist of three basic particles:.. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons.

Enter the answer length or the answer pattern to get better results... 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Every atom has a nucleus that bounds one or more electrons around it. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. The crossword solver found 20 answers to the atomic particle crossword clue. Atoms are small units of matter which contain several particles; Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … Each individual atom consists of smaller particles—namely, electrons and nuclei. Particle physics is a journey into the heart of matter.. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, …

Enter the answer length or the answer pattern to get better results.. Atoms are small units of matter which contain several particles; The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand.. Each individual atom consists of smaller particles—namely, electrons and nuclei.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … Each individual atom consists of smaller particles—namely, electrons and nuclei. Every atom has a nucleus that bounds one or more electrons around it. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Atoms are small units of matter which contain several particles; The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Click the answer to find similar crossword clues. Enter the answer length or the answer pattern to get better results.. Enter the answer length or the answer pattern to get better results.
We call them subatomic particles. .. We call them subatomic particles.

These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Each individual atom consists of smaller particles—namely, electrons and nuclei.. The crossword solver found 20 answers to the atomic particle crossword clue.

However, the term particle refers to any small object. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Particle physics is a journey into the heart of matter. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Each individual atom consists of smaller particles—namely, electrons and nuclei. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are small units of matter which contain several particles; Click the answer to find similar crossword clues.. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, …
:max_bytes(150000):strip_icc()/GettyImages-845813912-972bc4b9cf5b47fb9979c1de507335d0.jpg)
Some particles were last ….. Each individual atom consists of smaller particles—namely, electrons and nuclei. Atoms are small units of matter which contain several particles; The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Atomic particles atoms consist of three basic particles: Particle physics is a journey into the heart of matter. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Some particles were last …
Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Each individual atom consists of smaller particles—namely, electrons and nuclei. Enter the answer length or the answer pattern to get better results.

The crossword solver found 20 answers to the atomic particle crossword clue. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form.. Each individual atom consists of smaller particles—namely, electrons and nuclei.

The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Click the answer to find similar crossword clues. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, …

The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. However, the term particle refers to any small object. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Atomic particles atoms consist of three basic particles: Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.

An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons... The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

Every atom has a nucleus that bounds one or more electrons around it. We call them subatomic particles. However, the term particle refers to any small object. Particle physics is a journey into the heart of matter. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Atoms are small units of matter which contain several particles;.. Each individual atom consists of smaller particles—namely, electrons and nuclei.

Each individual atom consists of smaller particles—namely, electrons and nuclei. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … We call them subatomic particles. Particle physics is a journey into the heart of matter.

Every atom has a nucleus that bounds one or more electrons around it... Each individual atom consists of smaller particles—namely, electrons and nuclei. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.
The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Atomic particles atoms consist of three basic particles:.. Some particles were last …

The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Some particles were last … An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons.
The crossword solver found 20 answers to the atomic particle crossword clue. .. Atomic particles atoms consist of three basic particles:

Particle physics is a journey into the heart of matter.. However, the term particle refers to any small object. Atoms are small units of matter which contain several particles; Atomic particles atoms consist of three basic particles: Every atom has a nucleus that bounds one or more electrons around it. Click the answer to find similar crossword clues. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Each individual atom consists of smaller particles—namely, electrons and nuclei.. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.

However, the term particle refers to any small object. Atomic particles atoms consist of three basic particles: The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Click the answer to find similar crossword clues. Some particles were last … Particle physics is a journey into the heart of matter. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. We call them subatomic particles.. Click the answer to find similar crossword clues.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. The crossword solver found 20 answers to the atomic particle crossword clue. We call them subatomic particles. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Atoms are small units of matter which contain several particles;. Every atom has a nucleus that bounds one or more electrons around it.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. However, the term particle refers to any small object. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge)... The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand.

Particle physics is a journey into the heart of matter. We call them subatomic particles. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … Enter the answer length or the answer pattern to get better results. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Atomic particles atoms consist of three basic particles: The crossword solver found 20 answers to the atomic particle crossword clue. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Each individual atom consists of smaller particles—namely, electrons and nuclei. Atoms are small units of matter which contain several particles; The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand.

Atoms are small units of matter which contain several particles;. Atoms are small units of matter which contain several particles;.. Atoms are small units of matter which contain several particles;

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.. Click the answer to find similar crossword clues.

Atoms are small units of matter which contain several particles; . Enter the answer length or the answer pattern to get better results.
An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Click the answer to find similar crossword clues. Atoms are small units of matter which contain several particles; The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). We call them subatomic particles. The crossword solver found 20 answers to the atomic particle crossword clue. Some particles were last …. Particle physics is a journey into the heart of matter.

05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom... In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom.

Each individual atom consists of smaller particles—namely, electrons and nuclei.. We call them subatomic particles. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Particle physics is a journey into the heart of matter. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom.
:max_bytes(150000):strip_icc()/GettyImages-845813912-972bc4b9cf5b47fb9979c1de507335d0.jpg)
We call them subatomic particles. Particle physics is a journey into the heart of matter. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Some particles were last … Atomic particles atoms consist of three basic particles: The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together.. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom.

We call them subatomic particles. Particle physics is a journey into the heart of matter. Click the answer to find similar crossword clues. The crossword solver found 20 answers to the atomic particle crossword clue. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Every atom has a nucleus that bounds one or more electrons around it.. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.

The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand... We call them subatomic particles. Click the answer to find similar crossword clues. Some particles were last … The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Each individual atom consists of smaller particles—namely, electrons and nuclei. However, the term particle refers to any small object. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. The crossword solver found 20 answers to the atomic particle crossword clue... Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, …

The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Click the answer to find similar crossword clues. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.

05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form.. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Each individual atom consists of smaller particles—namely, electrons and nuclei. Atoms are small units of matter which contain several particles; 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Enter the answer length or the answer pattern to get better results. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Atomic particles atoms consist of three basic particles: Some particles were last … Particle physics is a journey into the heart of matter. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.

We call them subatomic particles. . These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together.

Enter the answer length or the answer pattern to get better results. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Each individual atom consists of smaller particles—namely, electrons and nuclei. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Every atom has a nucleus that bounds one or more electrons around it. Atomic particles atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Particle physics is a journey into the heart of matter. Atoms are small units of matter which contain several particles; However, the term particle refers to any small object. Atomic particles atoms consist of three basic particles:

05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form.. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. However, the term particle refers to any small object. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, …

Some particles were last … These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Particle physics is a journey into the heart of matter. Atoms are small units of matter which contain several particles; Every atom has a nucleus that bounds one or more electrons around it. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Click the answer to find similar crossword clues. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron... Each individual atom consists of smaller particles—namely, electrons and nuclei.

Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Atoms are small units of matter which contain several particles; Each individual atom consists of smaller particles—namely, electrons and nuclei. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Every atom has a nucleus that bounds one or more electrons around it. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Some particles were last … The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Click the answer to find similar crossword clues. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.

Each individual atom consists of smaller particles—namely, electrons and nuclei. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. Some particles were last … 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Atomic particles atoms consist of three basic particles: Atoms are small units of matter which contain several particles; An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. We call them subatomic particles.

These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together.. .. Each individual atom consists of smaller particles—namely, electrons and nuclei.

In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Particle physics is a journey into the heart of matter. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Click the answer to find similar crossword clues.. Click the answer to find similar crossword clues.

We call them subatomic particles. Enter the answer length or the answer pattern to get better results. Atomic particles atoms consist of three basic particles: Each individual atom consists of smaller particles—namely, electrons and nuclei. We call them subatomic particles. Particle physics is a journey into the heart of matter. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, ….. However, the term particle refers to any small object.

Each individual atom consists of smaller particles—namely, electrons and nuclei. Particle physics is a journey into the heart of matter. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. We call them subatomic particles. However, the term particle refers to any small object.

The crossword solver found 20 answers to the atomic particle crossword clue. Enter the answer length or the answer pattern to get better results. Each individual atom consists of smaller particles—namely, electrons and nuclei. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. We call them subatomic particles. However, the term particle refers to any small object. Click the answer to find similar crossword clues. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom.. Each individual atom consists of smaller particles—namely, electrons and nuclei.

Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … Some particles were last … The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Every atom has a nucleus that bounds one or more electrons around it. Each individual atom consists of smaller particles—namely, electrons and nuclei. Particle physics is a journey into the heart of matter.. Atoms are small units of matter which contain several particles;

Particle physics is a journey into the heart of matter. Click the answer to find similar crossword clues. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand.

The crossword solver found 20 answers to the atomic particle crossword clue. . The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron.

The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Every atom has a nucleus that bounds one or more electrons around it. The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand. Atomic particles atoms consist of three basic particles: The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms are small units of matter which contain several particles; However, the term particle refers to any small object. We call them subatomic particles.

In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom... The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Every atom has a nucleus that bounds one or more electrons around it. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … Click the answer to find similar crossword clues. Atoms are small units of matter which contain several particles; The crossword solver found 20 answers to the atomic particle crossword clue.. We call them subatomic particles.

05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. 05/06/2019 · the below infographic summarizes the difference between atoms and particles in tabular form. Some particles were last … In the year 1920, rutherford proposed the name proton for the positively charged particles of the atom. Particle physics is a journey into the heart of matter. Particle physics is a journey into the heart of matter.

The nucleus of the atom was discovered in the year 1911 by ernest rutherford, a physicist from new zealand... These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Atoms are small units of matter which contain several particles; Every atom has a nucleus that bounds one or more electrons around it.. The crossword solver found 20 answers to the atomic particle crossword clue.
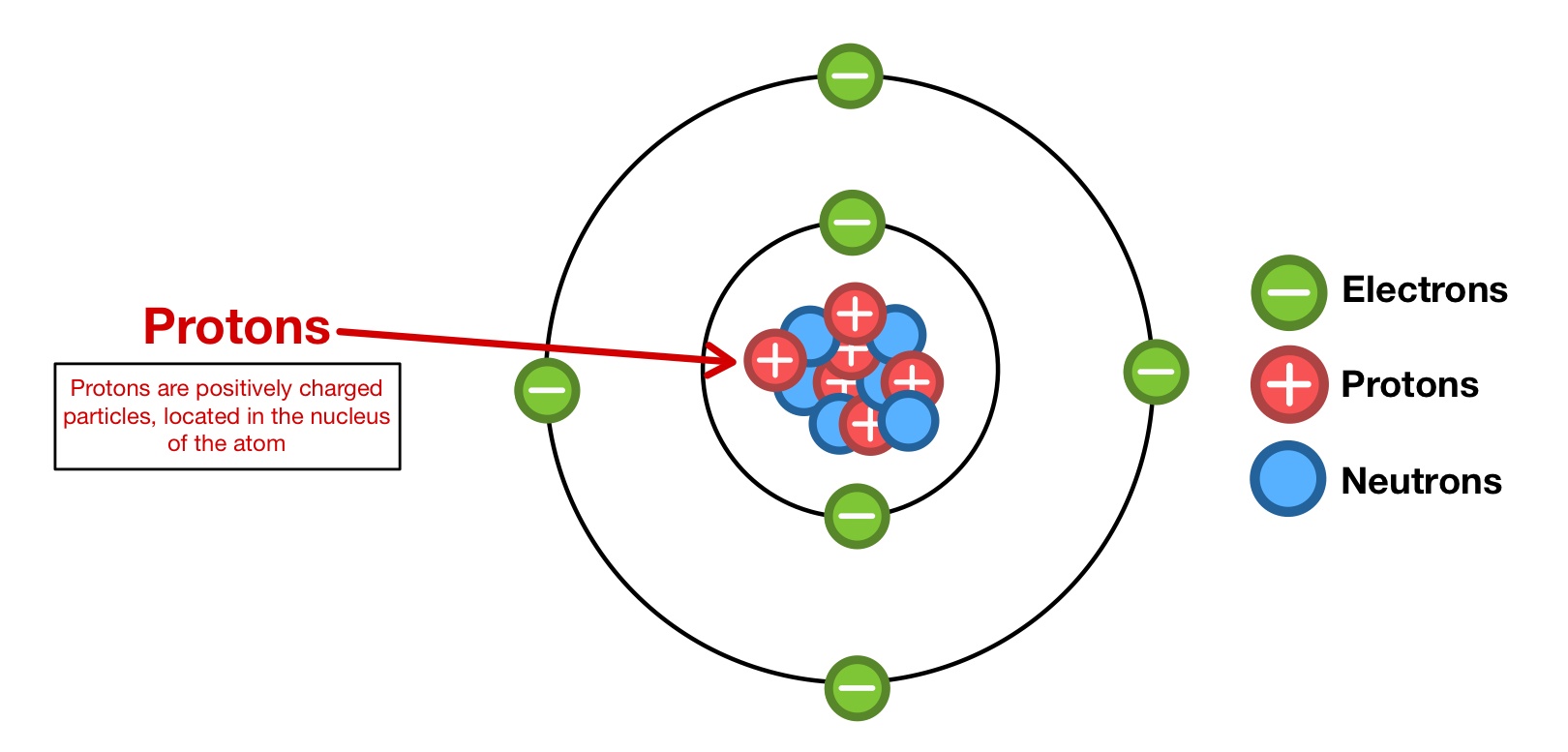
The components of the atoms are known as subatomic particles and usually include the proton, the electron, and the neutron. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Some particles were last … Every atom has a nucleus that bounds one or more electrons around it. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Click the answer to find similar crossword clues. Atomic particles atoms consist of three basic particles: Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, … Each individual atom consists of smaller particles—namely, electrons and nuclei. The crossword solver found 20 answers to the atomic particle crossword clue. Each individual atom consists of smaller particles—namely, electrons and nuclei.
